In response to the current worldwide pandemic, CytoDyn (OTCQB:CYDY) is frenetically tracking down an FDA approval for its monoclonal antibody, leronlimab in treatment of COVID-19. I was initially quite skeptical of this quest, but I have slowly warmed to the prospect.
As matters now stand, I am waiting and watching with guarded optimism as it goes through the early stages of revealing data from its COVID-19 clinical trials. This article discusses the current status of CytoDyn’s COVID-19 quest and explains my bullish outlook for CytoDyn, albeit an outlook that recognizes daunting challenges.
CytoDyn has two COVID-19 trials in progress, one a phase 2 for mild-to-moderate which is wrapping up; the other, a phase 3 that is enrolling patients with preliminary data expected later this year
CytoDyn has amassed an interesting track record in COVID-19 treatment that few can match. In order to avoid repetition, let me cite my suite of CytoDyn COVID-19 articles. The one article that I will use as a base of reference for this current posting is “CytoDyn: COVID-19 Crunch Time” (“Crunch Time”).
Crunch Time sets out a detailed review of the 75 patients along with their outcomes that were treated under CytoDyn’s highly successful EIND COVID-19 program. This is the program that has legitimized CytoDyn’s prospects in COVID-19. It has gotten the attention of important COVID-19 therapists such as Drs. Otto Yang and Bruce Patterson.
As described in Crunch Time, the FDA instructed CytoDyn to discontinue this EIND program. It was to direct its attention to its two registered COVID-19 clinical trials, its:
- CD10, a phase 2 mild-to-moderate trial, ID NCT04343651 and
- CD12, a phase 2b/3 severe or critical trial NCT04347239.
CD10, the mild-to-moderate trial, was fully enrolled, actually treating 84 patients rather than the 75 initially set out on clinicaltrials.gov. These 84 patients were then divided into two groups, with 28 in a placebo group and 56 treated with two weekly doses of 700 mg leronlimab.
The company has unblinded the data and is currently evaluating it. This process of releasing the data is creating the tribulations referenced in the title to this article as will be explained below.
Patients in CD10 have completed their prescribed two weeks of treatment; the trial data is unblinded, and CytoDyn has begun releasing safety data
As can be gleaned from multiple Seeking Alpha comment streams, CytoDyn investors, bulls, bears, and kibitzers are a garrulous, excitable, and occasionally quarrelsome lot. These characteristics have been amplified in recent weeks as CytoDyn stumbles through challenging FDA drug approval processes. It has had nothing but headaches in applying for approval for leronlimab in its lead indication for the treatment of HIV.
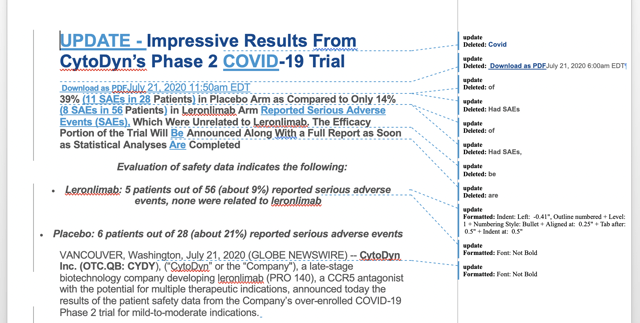
Now, as it begins to release data from CD10 in treatment of mild-to-moderate COVID-19, its challenges are continuing unabated. Take, for example, its initial results press release issued in the early hours of 7/21/20 headlined:
Then, a few hours later, it issued the following updated press release:
I imported these two press releases into Word and ran comparisons. The most notable changes are in the excerpt below:
Some may agree with me that the second release was unnecessary. While it corrected slight errors and added useful details to the heading, it also was disrespectful of those who had to understand what was going on. More than anything else, it disclosed CytoDyn as a slipshod organization, publishing an important press release before it was fully proofed and vetted.
So, what about the substance of the updated press release? It is a bit of a head scratcher as well. CD10 was designed to evaluate the safety and the efficacy of leronlimab in treatment of mild-to-moderate COVID-19. CytoDyn’s long time CEO Nader Pourhassan [NP] has been pushing for rapid-fire unblinding and release of data since early July.
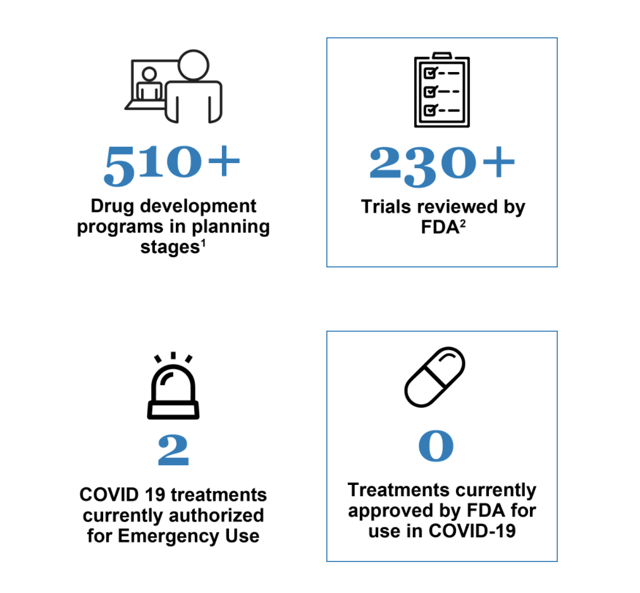
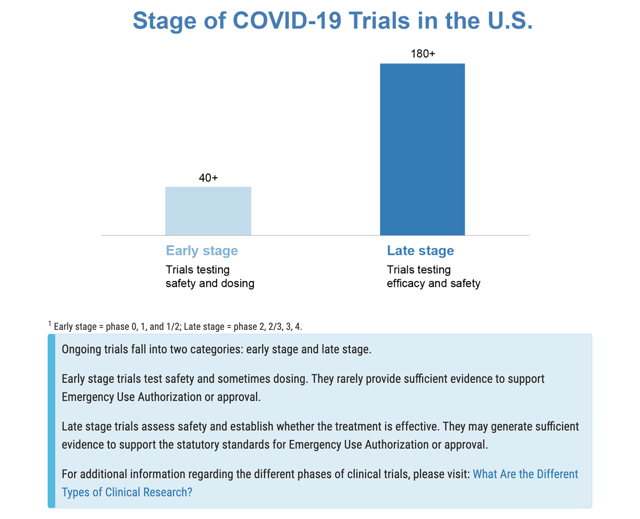
This urgency is unfortunate but understandable. There is a raft of therapeutics being reviewed in treatment of COVID-19 as discussed in detail on the FDA’s Coronavirus Treatment Acceleration Program [CTAP] website. Little CytoDyn with its leronlimab is just one of hundreds seeking the brass ring of FDA approval as shown by the CTAP graphic below:
CytoDyn is well beyond the planning stage; however, it is far from alone in this respect either as shown by this additional CTAP graphic:
There are a number of huge pharmaceutical companies in the hunt. These include Regeneron (REGN), Eli Lilly (LLY), and others. Such competition has access to funding which permits it to fund larger trials which command widespread media and FDA attention such as Gilead (GILD) achieved with remdesivir.
This is the background that CytoDyn is facing when it pursues leronlimab as a COVID-19 therapeutic. It is not easy for it to get attention. NP knows that CytoDyn needs positive data to break through. He was no doubt elated to learn that CD10’s SAEs outperformed placebo and was hoping that the market would share his enthusiasm. It did not.
In order for CD10 to attract positive attention, it will have to actually perform an analysis that shows it achieving a statistically significant level of efficacy. During CytoDyn’s 7/22/20 special meeting of shareholders, NP noted that patients typically recover from mild-to-moderate COVID-19 (8:18/30:03).
NP framed the question for this population as relating to adverse events during recovery. He considers the safety data reported in CytoDyn’s press release as signifying that leronlimab provides a 64% reduction of SAEs during recovery. This interpretation is controversial.
Regardless, he recognizes that CD10’s data must undergo a complete statistical analysis. The statistical team at Amarex, CytoDyn’s CRO, has been tasked with providing a top line report. He notes that he wants the report within days; he recognizes such analysis usually takes six weeks.
NP is characteristically optimistic on his time lines and ultimate data outcomes, so I am learning to take his pronouncements as best case scenarios, particularly when they are date related. He notes that CytoDyn could be just a few weeks from filing for an emergency approval for leronlimab in the treatment of COVID-19 (10:26-11:07/30:03).
CD12 is still undergoing enrollment, although an initial cohort of 100 patients who have completed their course of treatment will be presented for data safety monitoring committee review shortly
CD10 has a far more complicated primary outcome measure than does CD12. CD10 requires a multifactorial evaluation per its clinicaltrials.gov description.
Primary Outcome Measures:
- Clinical Improvement as assessed by change in total symptom score (for fever, myalgia, dyspnea and cough) [Time Frame: Day 14]
Note: The total score per patient ranges from 0 to 12 points. Each symptom is graded from 0 to 3. [0=none, 1=mild, 2=moderate, and 3=severe]. Higher scores mean a worse outcome.
On the other hand, the Primary outcome measure for CD12 is grimly simple; clinicaltrials.gov lists it as follows:
Primary Outcome Measures:
- All-cause mortality at Day 28 [Time Frame: Day 28]
Day 0 refers to the data of randomization/first treatment.
CD12 is a larger trial; rather than CD10’s target population of 75 patients, CytoDyn is working to enroll 390 patients in CD12. A full 100 patients have completed their four-week course of leronlimab called for in CD12, as opposed to two weeks for CD10.
Both CD10 and CD12 have diverse secondary outcome measures, with CD10 having twelve, to CD12’s four. Accordingly, a full data analysis for CD10 will include analyzing reports from the various hospitals conducting trials on a wide variety of data points. Can this be done completely and accurately within an expedited time window?
Perhaps, it can. To me, it reeks of just the type of unfortunate result described in “CytoDyn’s BLA Blues“. We shall see. On 7/27/20, CytoDyn pumped out a new press release per Seeking Alpha news report headlined below:
The goal for this conference is:
…to provide a comprehensive update on the Company’s two trials for COVID-19 patients with mild-to-moderate and severe-to-critical indications.
For CD10 bulls, a home run would report statistically significant data showing positive effect of leronlimab on mild-to-moderate COVID-19. That is my hope, but my expectations are more moderate. I question whether the trial studied enough patients to reach the level of statistical significance.
What are we likely to hear about CD12? During the 7/22/20 investor’s conference, NP noted that a data safety monitoring committee [DMC] will be performing a safety review of the first 100 patients. He notes the distinction between this and the full interim review. The DMC report goes only to the FDA for its determination as to whether the trial should continue (28:00-28:30/30:03).
As of CytoDyn’s 7/22/20 investors’ conference, CytoDyn reported that it had enrolled 153 patients in CD12; this compares to the 152 enrolled on 7/18/20 during NP’s interview with Dr. Been. Once it has 195, it intends to perform an interim analysis.
It is unclear to me whether NP envisions running its CD12 interim analysis once 195 patients are enrolled, which is what he seems to say. It would make more sense to me to run such an analysis after the 195 patients have completed 4 weeks of treatment. When I check the published protocol for CD12, I found no answer to this, nor any provision for unblinding data to allow any such analysis.
Perhaps one or more comments will help with this. It is a potentially important point because NP has set this interim analysis date as a possible date when good data might merit an FDA approval.
Conclusion
CytoDyn investors have been acutely focused on the time line for receipt of statistically significant data to support the approval of leronlimab as a potent therapeutic in treatment of COVID-19. In “CytoDyn’s BLA Blues“, hundreds of the comments focused on timing of significant CytoDyn COVID-19 data. With such a diverse group of comments there was no consensus on the timeline for data.
The article itself focused not on COVID-19 but rather on CytoDyn’s misadventures in filing its BLA for leronlimab in treatment of HIV as a combination therapy. I did mention the much anticipated COVID-19 data insofar as it is the material driver of my bullish CytoDyn thesis.
Please note I am bullish because I anticipate good data from CytoDyn’s COVID-19 trials based on Dr. Patterson’s analysis as discussed in “CytoDyn’s COVID-19 Game Changer” as follows:
Dr. Patterson notes that an effective COVID-19 therapy must fulfill three roles:
- it must quiet the cytokine storm,
- it must restore the immune response and
- it must shut off the virus (20:40-21.02/3:08:37).
Dr. Fauci and others have suggested this may take two drugs. Leronlimab accomplished all three. After seeing results from hundreds of patients over multiple weeks of therapy, Dr. Patterson is optimistic. He has observed that leronlimab may stop COVID from transforming from a viral disease into an immunological disease if administered early enough, and if administered later, repair damage from immunosuppression (22:05-23.20/3:08:37).
Dr. Patterson’s work has moved me from a skeptical neutral position on CytoDyn to a cautiously bullish stance. I remain optimistic in my expectations for good COVID-19 data. I am substantially tempering my expectations of the date we might receive such data in any definitive form.
Despite the abuse it engenders, I will repeat the following caution from my previous article:
… CytoDyn is an exciting stock with barrels of potential. It is also among the riskiest of all potential investments, an OTC biotech with no income and few assets. Unless you are an experienced investor in this area you should shy away, or if you invest be sure you can afford a complete loss.
In writing this article, I have been greatly assisted by my review of TranscriptShare transcriptions of recent CytoDyn presentations; my study included NP’s 7/18/22 interview with Dr. Been and CytoDyn’s 7/22/20 investor presentation. It is far easier to study and run searches on transcripts than it is of oral presentations.
Disclosure: I am/we are long CYDY GILD. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: I may buy or sell shares in CYDY and GILD over the next 72 hours.
|
|





Leave a Reply
You must be logged in to post a comment.